Flexible biomanufacturing capacity for
Biologics
End-to-end Expertise for Biologics Facilities
End-to-end Expertise for Biologics Facilities
At KeyPlants, we provide comprehensive services for biomanufacturing facility design, construction, and commissioning from concept to full operational status. Our expert team specializes in process design, automation, facility design, and project management, with deep expertise in modular offsite construction to enhance scalability and flexibility.
A growing demand for flexible biomanufacturing capacity is shaping the industry, driven by advancements in personalized medicine, high-titer biologics, regionalized production, and supply chain risk mitigation. Sustainability also plays a key role, influencing the development of biomanufacturing facility construction strategies that optimize efficiency and minimize environmental impact.
Our approach focuses on developing scalable, flexible solutions that align with client-specific production needs while maintaining efficiency, quality, and compliance. By leveraging modular facility design, we ensure faster project timelines, cost predictability, and seamless integration of process equipment and automation systems.
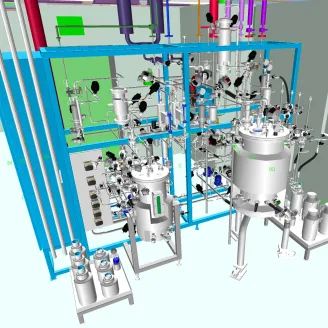
Flexible Biomanufacturing Capacity Through Modular and pods
In biologics production, processes typically include cell culture or microbial fermentation, followed by downstream purification, formulation and filling. In addition, viral vector technologies are increasingly used in gene therapy and advanced biologics manufacturing.
To meet the evolving needs of biologics manufacturers, facilities must prioritize flexible biomanufacturing capacity. Modular facilities and pods enable scalability and the opportunity to repurpose or even move facilities.

Production Techniques for Biomanufacturing
Production Techniques for Biomanufacturing
Biologics are complex drugs manufactured using living organisms such as mammalian and microbial cells or gene therapy vectors. Below are the main methods biologics drug substance production:
Mammalian Cell Culture
This widely used technique is essential for producing biologics, including monoclonal antibodies. Large-scale bioreactors cultivate mammalian cells, genetically modified to produce target proteins. The cells are harvested and purified to obtain the final drug substance.
Microbial Fermentation
Using bacteria or yeast, this technique is crucial for producing proteins such as insulin and vaccines. The microorganisms are grown in large-scale bioreactors with a specific nutrient-rich medium. Afterward, the protein is extracted, purified and formulated.
Viral Vector Manufacturing
Viral vectors are used to deliver gene therapies, vaccines and immunotherapies. In this case, a virus or virus-like particle is modified and then used to deliver therapeutic payloads into target cells.
The main production steps are listed below. By working with KeyPlants for your facility design you ensure flexible biomanufacturing capacity today and for future needs.
- Cell line development – Optimizing conditions for productive cell growth
- Cell culture phase – Growing modified cells in bioreactors under controlled conditions
- Harvesting – Extracting the product from the cell culture
- Purification – Separating the product from cell debris and contaminants
- Formulation – Preparing the final dosage form
- Filling - Aseptic filling of the formulated final product
As biologics manufacturing technologies evolves, investing in flexible capacity for biomanufacturing becomes increasingly important. Ensuring adaptable and scalable facilities is going to be critical when designing biomanufacturing facilities for tomorrow.
Biomanufacturing Facility Design & Construction
KeyPlants provides end-to-end solutions for biomanufacturing facility design and construction, supporting biologics production at every stage.
We design facilities optimized for biologics manufacturing, including cell culture, microbial fermentation, viral vector production and aseptic filling. By implementing cutting-edge bioprocessing technology and automation, we ensure that facilities remain compliant, scalable, and future-ready.

FAQ
What makes modular biomanufacturing facilities a smart investment?
Modular biomanufacturing facilities and pods reduce project time schedule, increase predictability and provide adaptable production spaces for biologics. KeyPlants specializes in offsite facility design and construction, helping clients establish state-of-the-art biologics manufacturing sites with enhanced flexibility.
How does KeyPlants support biomanufacturing facility design?
KeyPlants designs biomanufacturing facilities starting from process design and optimization. Our expertise in facility layout, cleanroom integration, and automation ensures the deployment of efficient biologics production facilities.
What are the key challenges in biologics facility design?
Challenges include meeting stringent industry regulations, ensuring sterility, and optimizing facility workflows. With decades of experience, KeyPlants delivers turnkey facility solutions that meet GMP requirements while accelerating project timelines.
Why is scalable facilities essential in biologics manufacturing?
Scalability ensures that biomanufacturing facilities can handle evolving production demands, including new modalities or updated capacity requirements. KeyPlants provides modular and flexible facility solutions that allow biologics manufacturers to expand operations seamlessly.
How does flexible biomanufacturing capacity benefit biologics production?
Flexible biomanufacturing capacity through offsite construction allows biologics manufacturers to scale production efficiently, adapt to new therapies, and optimize facility utilization. By integrating modular designs and single-use systems, KeyPlants enables faster facility deployment while maintaining a high quality and regulatory compliance.
Related Pages