Pharmaceutical Facility Design
Pharmaceutical facility design is at the heart of our expertise—ensuring your facility meets the highest GMP standards while enabling efficient, scalable production. We specialize in the design of pharmaceutical manufacturing plants that align with your specific process needs, from early concept to detailed engineering.
Whether you're building a new pharmaceutical factory or upgrading an existing plant layout, our pharmaceutical facility engineering team delivers compliant, flexible solutions.
What We Deliver in Facility Design
GMP-Compliant Facility Design
We design pharmaceutical facilities that meet the highest GMP and regulatory standards, ensuring global compliance.
Cleanroom & Containment Design
Tailored cleanroom and containment environments that support sterile production and high-potency manufacturing while minimizing contamination risks.
Process & Utility Flow Optimization
Smart facility layouts that optimize the flow of personnel, materials, waste, and utilities to support efficient, safe, and compliant operations.
Modular & Scalable Design Solutions
Our modular approach enables rapid deployment and flexible scaling—delivering high-performance facilities designed to grow with your needs.
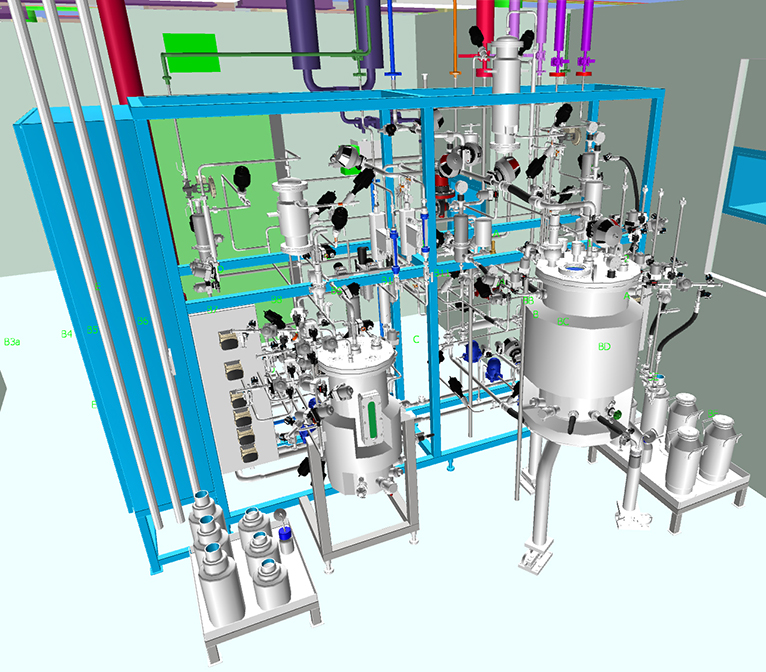
Equipment Integration Planning
Designs developed in close alignment with user requirements and equipment specifications to streamline installation, and enhance operability.
Cross-Functional Engineering Expertise
A multidisciplinary team combining process, automation, HVAC, architectural, structural and electrical expertise to deliver fully integrated facility solutions.
Pharmaceutical Facility Design Backed by Global Experience
We design facilities for pharmaceutical and biotech companies. From small-scale labs to commercial production plants, our team brings deep GMP experience and engineering expertise to every project. Each design is tailored to your products and manufacturing process.

A Structured Approach to GMP Facility Design
Our design process is built around your manufacturing goals. We begin by understanding your product and process, then apply a cross-functional approach combining process and utility, automation, HVAC and cleanroom expertise. The result is a fully integrated facility design - optimized for flow, compliant with global regulations, and ready for implementation.

Flexible Facility Design Service for Modular or Traditional Construction
Our pharmaceutical facility design service includes all key elements to move your project from concept to construction readiness. At feasibility and concept stages we develop layout and zoning plans suitable for modular offsite construction, but equally suitable for traditional design and build.
Starting your facility design with concept development by KeyPlants gives you the flexibility to later decide for modular or conventional construction with no re-work of the facility design needed.

FAQ
FAQ
What types of pharmaceutical facilities do KeyPlants design?
We design a wide range of GMP-compliant pharmaceutical facilities, including fill-finish plants, biologics manufacturing sites, oral solid dosage facilities, labs, and cleanrooms for both clinical and commercial use.
Do you provide both conceptual and detailed design?
Yes. We support projects from early concept development through basic and detailed design, delivering layouts, technical specifications, cleanroom zoning, and documentation tailored to your regulatory and operational needs.
Can your designs accommodate modular construction?
Absolutely. Modular thinking is part of our DNA. Our facility designs are optimized for off-site construction when applicable, reducing timelines, improving quality, and enabling faster scale-up.
How do KeyPlants ensure GMP compliance in your designs?
We take a fully integrated approach — aligning facility layout with process equipment needs and automation strategies to streamline implementation and operational efficiency.
We take a fully integrated approach — aligning facility layout with process equipment needs and automation strategies to streamline implementation and operational efficiency.
All our designs are developed in accordance with GMP, FDA, EMA, and we follow ISPE guidelines. Our engineering teams work closely with quality and validation experts to ensure full compliance from the start.
Do KeyPlants integrate process equipment and automation in the design?
Yes. Automation enhances process consistency, reduces human error, and improves data integrity in pharmaceutical engineering process design. Advanced monitoring and control systems ensure real-time tracking, facilitating predictive maintenance, increased efficiency, and seamless regulatory compliance.
Our Projects