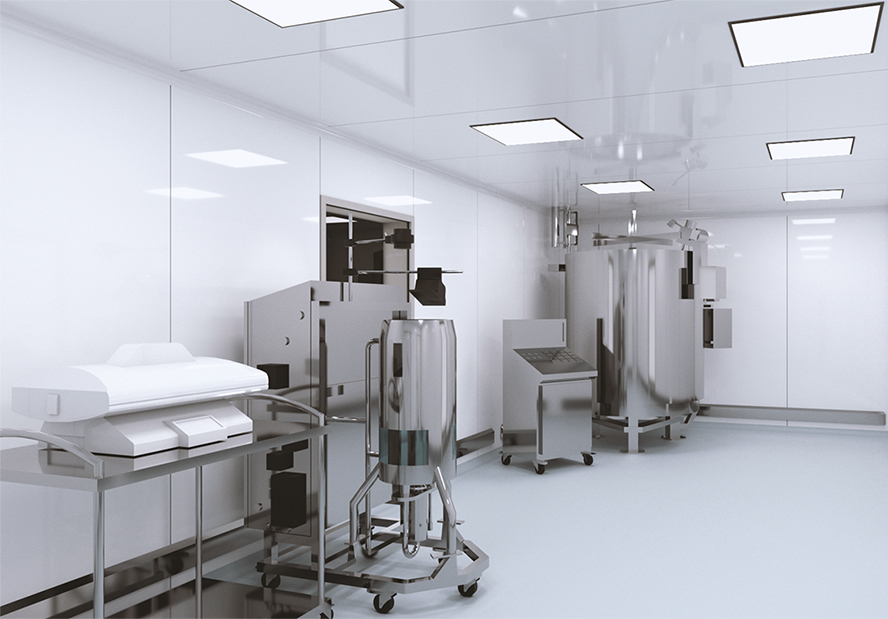
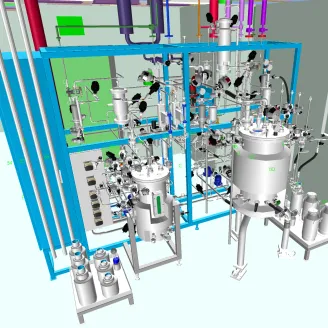
Pharmaceutical Cleanroom Design
Pharmaceutical cleanroom design is essential for maintaining product integrity, ensuring regulatory compliance, and enabling safe pharmaceutical production. At KeyPlants, we design and deliver cleanroom environments that meet the highest GMP standards, tailored to your specific process, facility, and scalability needs.
What We Deliver in Pharmaceutical Cleanroom Design
GMP-Compliant Cleanroom Design
We deliver cleanrooms that meet global GMP standards, supporting safe, compliant, and high-quality pharmaceutical production.
Modular Cleanroom Design & Delivery
Our prefabricated cleanrooms reduce timelines and enable fast deployment with minimal disruption to your ongoing operations.
ISO Classification & Zoning Strategy
We define ISO classes, zoning, and pressure regimes to maintain strict environmental control tailored to your process requirements.
HVAC & Environmental System Design
We integrate HVAC, temperature, humidity, and pressure control to ensure stable, validated, and energy-efficient cleanroom conditions.
Contamination Control & Process Flow
Our layouts are designed to minimize contamination through optimized flows for personnel, materials, and waste management.
Fully Integrated Design & Engineering
We manage the full scope of cleanroom design - from initial concept through detailed engineering and documentation.
Cleanroom Design for Critical Environments
We specialize in pharmaceutical cleanroom design for advanced, regulated environments. Our expertise spans from CNC and Class D spaces, through to aseptic production and high-containment cleanrooms, always tailored to meet GMP and FDA/EMA requirements. Every design ensures precise airflow control, temperature and humidity stability, and minimized contamination risk - supporting the highest levels of product and patient safety.

Tailored Solutions for Every Manufacturing Process
We understand that no two manufacturing processes are identical. That’s why our cleanroom designs are fully adapted to your production flow, spatial constraints, and regulatory framework. Whether you need unidirectional personnel and material flow, zoning strategies, or scalable layouts for future expansion, we collaborate closely with your team to ensure a cleanroom that fits both your operations and long-term strategy.

Seamless Integration with Facility & Process
Our cleanroom solutions are never designed in isolation. We align cleanroom layouts with the full facility design and production process to ensure functionality, compliance, and cost-efficiency. This integrated approach simplifies engineering, speeds up project delivery, and reduces on-site risks - whether the cleanroom is part of a greenfield facility or integrated into an existing production environment.
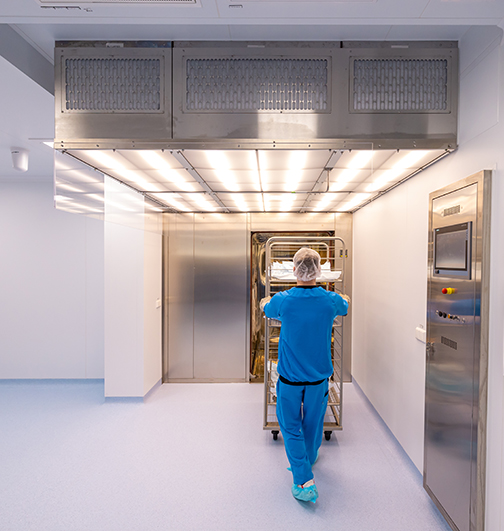
Explore our Modular Cleanroom pods
Our pre-manufactured cleanroom pods combine the same GMP-design expertise you rely on, with a modular platform built for rapid deployment, scalability and reuse. Whether you’re expanding capacity, adapting to new processes or entering a new market, our pod solutions install within weeks, integrate seamlessly with existing infrastructure and minimise downtime.

FAQ
FAQ
What is pharmaceutical cleanroom design?
Pharmaceutical cleanroom design involves creating controlled environments that meet strict cleanliness and environmental standards for drug manufacturing. This includes regulating temperature, humidity, air pressure, and particle levels in compliance with GMP and ISO classifications.
What cleanroom standards does KeyPlants design to?
KeyPlants designs cleanrooms in compliance with international standards such as EU GMP Annex 1, ISO 14644, and FDA cGMP. Our solutions are tailored to meet the specific regulatory requirements of your production processes and target markets.
Can cleanrooms be designed for both new and existing facilities?
Yes. We offer cleanroom designs for both greenfield and brownfield projects. Our modular approach makes it possible to integrate new cleanrooms into existing buildings with minimal disruption and high design flexibility.
How does KeyPlants ensure contamination control in cleanroom design?
We implement proven zoning strategies, unidirectional flows, HEPA filtration systems, and proper material/personnel segregation to ensure contamination risks are minimized throughout the cleanroom environment.
Does KeyPlants offer turnkey delivery of cleanroom facilities?
Yes. In addition to design, KeyPlants provides complete turnkey delivery — from engineering and prefabrication to installation and commissioning. This ensures speed, cost control, and quality throughout the entire cleanroom lifecycle.
Our Projects