Pharmaceutical Engineering consultants
Consulting Services
We have the expertises that you seek!
We have the expertises that you seek!
We specialize in providing efficient solutions throughout the entire production chain in the life science industry. We match junior and senior consultants to your specific needs, we also pair their personalities with your corporate culture and project team. Our consultants, who work both in the project or consulting side, are supported by expertise from the whole organization.
When you work with us, you become a part of a creative and welcoming company where everyone has the opportunity to develop their skills. We also hope that you will learn as much from us as we will learn from you.
Automation
Commissioning, Qualification and Validation (CQV)
Containment Study
Facility Design
Process Design
Process Engineering
Process Risk Analysis
Process Simulation
Project Management
Quality Assurance
Site Master Planning

KeyPlants automation delivers a full range of automation services, from complete project delivery to on-site consultancy services. Based on our own quality systems and project development methodology, we offer complete application development, testing, quality assurance and documentation for your projects. This includes:
- System independent conceptual and basic automation designs
- Full Distributed Control Systems design and implementation
- Full PLC/SCADA solution design and implementation
- System upgrade/replacement
- Both batch and continuous system design
- Cabinet I/O Layout and design
- Site based consulting and troubleshooting support 15:54
- In-house development and test systems
The team at KeyPlants automation has extensive experience across multiple industries, with particular focus on GMP-regulated environments, such as pharmaceuticals and biotechnology. All of our consultants work according to established international standards and guidelines, including ISA S88, ISA S95 and GAMP. Our automation team also has extensive experience and skills with Emerson

Our CQV consultants are experienced in leading and performing DQ, FAT, SAT, IQ, OQ and PQ for process equipment, QC instruments, cleanrooms, computerized systems (CSV), IT systems and much more. We also help with planning and leading PV/PPQ, CV, SV and Periodic Review.
Our backgrounds from different life science companies gives us a solution-oriented outlook and an ability to see projects through from start to finish. Furthermore, we will help you meet the most firm quality, cost and schedule requirements.

Protecting employees from exposure to dangerous agents and ensuring a correct GMP environment for the product during the manufacturing process, often requires the use of a combination of isolation technologies, proper air classifications, room and isolator pressurisation schemes, and personal protective equipment.
When making a Containment Study, we will help you define the strategy and select the technology solution you need to protect personnel, the product, and the environment, as required for your production.
First, we will define the overall requirements and justifications in a Containment Strategy, and assess the available technologies that may be employed according to the specific requirement. The strategy will be based on your operations and the Operator Exposure Limit (OEL) for your product.
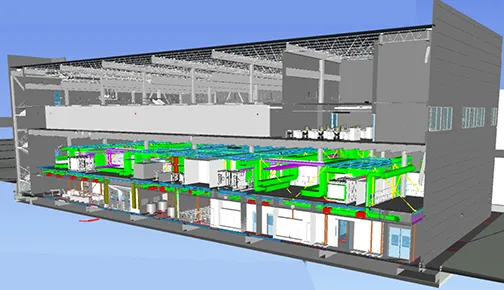
Our design managers draw on their practical experience to steer your project design around all possible challenges, avoiding unnecessary gold-plating, so you can meet your schedule and budgets. Product quality is always the top priority.
KeyPlants’ engineering experts are ready to assist you in ensuring your design and operation complies with all regulatory requirements. We are accustomed to working in an international environment, complying with local building codes and guidelines, and cooperating with local engineering firms all over the globe. We will perform efficient design reviews of your design in relation to GMP or other applicable regulations, based on extensive global experience.

Our engineers have extensive experience in process and plant design in the life science industry.
Through simulation, modeling and capacity analysis, we create robust and scalable solutions – from concept to finished production. Our process design includes Process Flow Diagram (PFD) and P&ID documentation, integration of automation and control systems and GMP-compliant solutions that ensure quality, efficiency and flexibility.

Our process engineers work with everything from preliminary studies and design to validation and daily operation. We offer technical support on equipments and processes as well as writing different process documentation such as change control, risk management and CAPA. Furthermore, we help with upgrades, process improvements and purchase of new equipment. By combining technical expertise with a deep understanding of production and regulatory requirements, we create processes that work in practice and provide long-term benefits.

At KeyPlants, SAFETY comes first. A plant failure that leads to personnel injuries, equipment and facility damage, or environmental implications, must always be prevented.
During the design development, our engineers can perform a safety risk analysis to identify all potential causes of failure, through a rigorous and systematic assessment of your facility, in order to find any weak points in your design.
We have the experience, and proven procedures in place, to perform an efficient risk analysis to identify the necessary measures needed to avoid, or mitigate, potential risks. Performing a safety study during the design phase will always pay off.

At KeyPlants, we offer process simulation and process optimisation studies for your batch or continuous processes. Process simulation is a computer model representing a unit operation, a process train, or an entire manufacturing plant.
As it’s a database model, you can quickly study process alternatives before risking capital expenditures. Proposals of optimised debottlenecking solutions for prioritised future plant requirements can be incorporated into the initial design, to achieve process flexibility and minimise overall capital cost.
Process Simulation can add value throughout the lifecycle of your new facility, from master planning debottlenecking and even day-to-day operations on existing site and operations.
We work with dynamic event-based plant simulation software tools that enable us, through efficient modelling efforts, to generate process operation timelines (Gantt charts) and resource histograms. When the model is developed, we have the expertise and experience to perform 'what-if' debottlenecking studies for the optimisation of complete trains, or equipment utilisation and the design of support systems.

Our technical project managers are experienced in leading and coordinating complex projects where technology, quality and regulatory requirements meet. We ensure that projects are carried out in a structured manner – from planning and design to implementation and handover – with a focus on time, budget and the right level of quality. We are able to lead small and large projects as well as support you with process administrators and controllers. By combining technical expertise with extensive project experience, we create clarity, reduce risks, and drive projects forward.

Our Quality Assurance team will support you, both in projects and regular production. We will work in line with your existing quality system, or we can help develop or expand your quality system to ensure compliance with current regulations. We can support you with different quality documents such as SOP, URS, CC, CAPA, deviations, improve processes as well as help you with product release and audits.
The QA team is backed by an organization with broad experience in pharmaceutical facility projects. We are used to see the full picture as well as make difficult decisions. Our technical experience also gives us a practical, solution-focused approach, and an ability to see projects through from beginning to end.

Proper site master planning is one of the most important factors in maximising return on capital investment and ensuring the long-term viability of a site.
When evaluating the feasability of a new project or site, or the refurbishment of an existing site/building, KeyPlants can provide valuable expertise to predict capital and manufacturing costs. We can support you in planning capital expenditures that will contribute to long-term financial success and maximise shareholder value.
Working in close collaboration with all stakeholders, we take critical issues such as site location, infrastructure, logistics and expandability into consideration. We also include the evaluation of energy, water usage, security, building location and layout, as well as production processes, Good Manufacturing Practice (GMP), material flow, warehousing, and personnel requirements, to create several concept options.
This results in an enhanced long-term site plan through the inclusion of multiple scheme scenarios. These contribute maximum cost effectivity and sustainability for our clients.
Automation

KeyPlants automation delivers a full range of automation services, from complete project delivery to on-site consultancy services. Based on our own quality systems and project development methodology, we offer complete application development, testing, quality assurance and documentation for your projects. This includes:
- System independent conceptual and basic automation designs
- Full Distributed Control Systems design and implementation
- Full PLC/SCADA solution design and implementation
- System upgrade/replacement
- Both batch and continuous system design
- Cabinet I/O Layout and design
- Site based consulting and troubleshooting support 15:54
- In-house development and test systems
The team at KeyPlants automation has extensive experience across multiple industries, with particular focus on GMP-regulated environments, such as pharmaceuticals and biotechnology. All of our consultants work according to established international standards and guidelines, including ISA S88, ISA S95 and GAMP. Our automation team also has extensive experience and skills with Emerson
Commissioning, Qualification and Validation (CQV)

Our CQV consultants are experienced in leading and performing DQ, FAT, SAT, IQ, OQ and PQ for process equipment, QC instruments, cleanrooms, computerized systems (CSV), IT systems and much more. We also help with planning and leading PV/PPQ, CV, SV and Periodic Review.
Our backgrounds from different life science companies gives us a solution-oriented outlook and an ability to see projects through from start to finish. Furthermore, we will help you meet the most firm quality, cost and schedule requirements.
Containment Study

Protecting employees from exposure to dangerous agents and ensuring a correct GMP environment for the product during the manufacturing process, often requires the use of a combination of isolation technologies, proper air classifications, room and isolator pressurisation schemes, and personal protective equipment.
When making a Containment Study, we will help you define the strategy and select the technology solution you need to protect personnel, the product, and the environment, as required for your production.
First, we will define the overall requirements and justifications in a Containment Strategy, and assess the available technologies that may be employed according to the specific requirement. The strategy will be based on your operations and the Operator Exposure Limit (OEL) for your product.
Facility Design

Our design managers draw on their practical experience to steer your project design around all possible challenges, avoiding unnecessary gold-plating, so you can meet your schedule and budgets. Product quality is always the top priority.
KeyPlants’ engineering experts are ready to assist you in ensuring your design and operation complies with all regulatory requirements. We are accustomed to working in an international environment, complying with local building codes and guidelines, and cooperating with local engineering firms all over the globe. We will perform efficient design reviews of your design in relation to GMP or other applicable regulations, based on extensive global experience.
Process Design

Our engineers have extensive experience in process and plant design in the life science industry.
Through simulation, modeling and capacity analysis, we create robust and scalable solutions – from concept to finished production. Our process design includes Process Flow Diagram (PFD) and P&ID documentation, integration of automation and control systems and GMP-compliant solutions that ensure quality, efficiency and flexibility.
Process Engineering

Our process engineers work with everything from preliminary studies and design to validation and daily operation. We offer technical support on equipments and processes as well as writing different process documentation such as change control, risk management and CAPA. Furthermore, we help with upgrades, process improvements and purchase of new equipment. By combining technical expertise with a deep understanding of production and regulatory requirements, we create processes that work in practice and provide long-term benefits.
Process Risk Analysis

At KeyPlants, SAFETY comes first. A plant failure that leads to personnel injuries, equipment and facility damage, or environmental implications, must always be prevented.
During the design development, our engineers can perform a safety risk analysis to identify all potential causes of failure, through a rigorous and systematic assessment of your facility, in order to find any weak points in your design.
We have the experience, and proven procedures in place, to perform an efficient risk analysis to identify the necessary measures needed to avoid, or mitigate, potential risks. Performing a safety study during the design phase will always pay off.
Process Simulation

At KeyPlants, we offer process simulation and process optimisation studies for your batch or continuous processes. Process simulation is a computer model representing a unit operation, a process train, or an entire manufacturing plant.
As it’s a database model, you can quickly study process alternatives before risking capital expenditures. Proposals of optimised debottlenecking solutions for prioritised future plant requirements can be incorporated into the initial design, to achieve process flexibility and minimise overall capital cost.
Process Simulation can add value throughout the lifecycle of your new facility, from master planning debottlenecking and even day-to-day operations on existing site and operations.
We work with dynamic event-based plant simulation software tools that enable us, through efficient modelling efforts, to generate process operation timelines (Gantt charts) and resource histograms. When the model is developed, we have the expertise and experience to perform 'what-if' debottlenecking studies for the optimisation of complete trains, or equipment utilisation and the design of support systems.
Project Management

Our technical project managers are experienced in leading and coordinating complex projects where technology, quality and regulatory requirements meet. We ensure that projects are carried out in a structured manner – from planning and design to implementation and handover – with a focus on time, budget and the right level of quality. We are able to lead small and large projects as well as support you with process administrators and controllers. By combining technical expertise with extensive project experience, we create clarity, reduce risks, and drive projects forward.
Quality Assurance

Our Quality Assurance team will support you, both in projects and regular production. We will work in line with your existing quality system, or we can help develop or expand your quality system to ensure compliance with current regulations. We can support you with different quality documents such as SOP, URS, CC, CAPA, deviations, improve processes as well as help you with product release and audits.
The QA team is backed by an organization with broad experience in pharmaceutical facility projects. We are used to see the full picture as well as make difficult decisions. Our technical experience also gives us a practical, solution-focused approach, and an ability to see projects through from beginning to end.
Site Master Planning

Proper site master planning is one of the most important factors in maximising return on capital investment and ensuring the long-term viability of a site.
When evaluating the feasability of a new project or site, or the refurbishment of an existing site/building, KeyPlants can provide valuable expertise to predict capital and manufacturing costs. We can support you in planning capital expenditures that will contribute to long-term financial success and maximise shareholder value.
Working in close collaboration with all stakeholders, we take critical issues such as site location, infrastructure, logistics and expandability into consideration. We also include the evaluation of energy, water usage, security, building location and layout, as well as production processes, Good Manufacturing Practice (GMP), material flow, warehousing, and personnel requirements, to create several concept options.
This results in an enhanced long-term site plan through the inclusion of multiple scheme scenarios. These contribute maximum cost effectivity and sustainability for our clients.

Talk to our sales representative for more information
FAQ
FAQ
What services do KeyPlants' pharmaceutical engineering consultants provide?
KeyPlants' pharmaceutical engineering consultants offer comprehensive solutions, including process- and facility design, automation, commissioning, qualification, validation (CQV), quality assurance (QA), and project management for the pharmaceutical and life sciences industries.
How do pharmaceutical engineering consultants ensure GMP compliance?
KeyPlants’ pharmaceutical engineering consultants follow strict Good Manufacturing Practice (GMP) guidelines during design, construction, and validation to meet regulatory requirements and ensure product quality and safety.
What industries do pharmaceutical engineering consultants work with?
KeyPlants' consultants primarily serve the pharmaceutical, biopharma, biotech, and medtech industries, delivering tailored engineering and validation solutions.
Why choose KeyPlants’ pharmaceutical engineering consultants?
KeyPlants’ consultants combine technical expertise with hands-on experience, ensuring projects are delivered on time, on budget, and fully compliant with global regulatory standards.
Can KeyPlants’ pharmaceutical engineering consultants lead large-scale projects?
Yes, KeyPlants’ consultants have extensive experience managing large-scale pharmaceutical projects, delivering end-to-end solutions that meet the highest quality and performance standards.
Experts On Call
Every company is different, with its own complexities and parameters. We offer a range of expertise both for large assignments, or for when you need an extra resource within your company or project team. Some of our more experienced consultants can also act as team leaders within your business.
Our specialist consultants work across the life sciences industry, including:
Specific expertise includes project management, QA, validation leadership, validation engineers, process engineers, and project leadership.

The KeyPlants Difference
Our consultants deliver work through project-based engagements in the life science industry. This makes all the difference when it comes to tackling challenges in a practical, hands-on manner. For a start, our consultants are not just specialists in their own fields, they also have experience working in cross-functional teams on our own projects. They work in a cross-functional way to get things done.
On the project side of KeyPlants' business, quality, safety, precision, agility, and sustainability are our main areas of focus. Because our consultants are experienced in these values, they bring the very same standards to your projects.
Our international experience means that as we've grown, we've evolved a distinctly multicultural mindset. Today, our consultants are comfortable working with teams of people from anywhere in the world. Our international experience also means that we are accustomed to working with regulators, government authorities and international agencies setting different standards and rules.

Work As a Consultant
We’re recruiting for a new hybrid project/consulting business that will offer expertise to the lifescience industry. Are you curious, adventurous, and ready for an amazing journey? If so, we offer an international work environment in which you can collaborate on interesting technical solutions that improve people’s lives.

Our consulting assignments
Our consulting assignments
KeyPlants developed the site master planning (SMP) for a new greenfield production site for peptide manufacturing. The project aims to meet the increasing demand for manufacturing capacity in a short- and long-term perspective. The SMP establishes how the site can support scalable production to meet the forecasted demand increase over a ten-year period. The plan features a phased expansion to ensure flexibility and optimized CAPEX. The facility will provide expandable production capacity with modular upstream and downstream production, warehousing, and central services. The developed plan is combining conventional and modular construction i.e., a hybrid solution. This hybrid design allows for efficient phased implementation while maintaining regulatory compliance.

KeyPlants supported with expertise in design verification and design assurance, with focus on requirements and risk management for a customer developing a new medical device for the worldwide market. The assignment requires expert knowledge in the design control process for medical devices as well as deep knowledge and understanding of regulatory requirements within the EU, the US and other regulated markets. The role includes risk management activities and to ensure that the design team understand and carry out project activities according to the required documentation, applicable SOP’s, and Design and Development Plan. The responsibility also includes creating and reviewing/approving project documentation throughout the design process. This work has led to a new medical device available on several markets worldwide.

KeyPlants has among other assignments provided Senior DeltaV SME’s for the client team to support the upgrade of a facility PCS. Our resources had roles as area leads and helped to drive the design and manage the delivery from the automation integrator. Moreover, KeyPlants has supported on-site C&Q of a new biologics facility, taking lead roles on the client team to drive the C&Q execution and support the DeltaV system on a technical level. In another assignment, we have provided expertise in Siemens PCS7 to develop new software in the DCS, where we helped to improve the effectiveness of the cooling systems in production.





