Design References
Design References
PiSA Pharmaceutical OSD Facility in Mexico meets Mexican, U.S. and EU GMP requirements
PiSA Pharmaceutical OSD Facility in Mexico meets Mexican, U.S. and EU GMP requirements
Project Details
Project Details
Client
PiSA Pharmaceutical
Location
Latin America
Market
Small Molecule
Solutions we provided
Design of a multi-product OSD facility
Project Overview
Project Overview
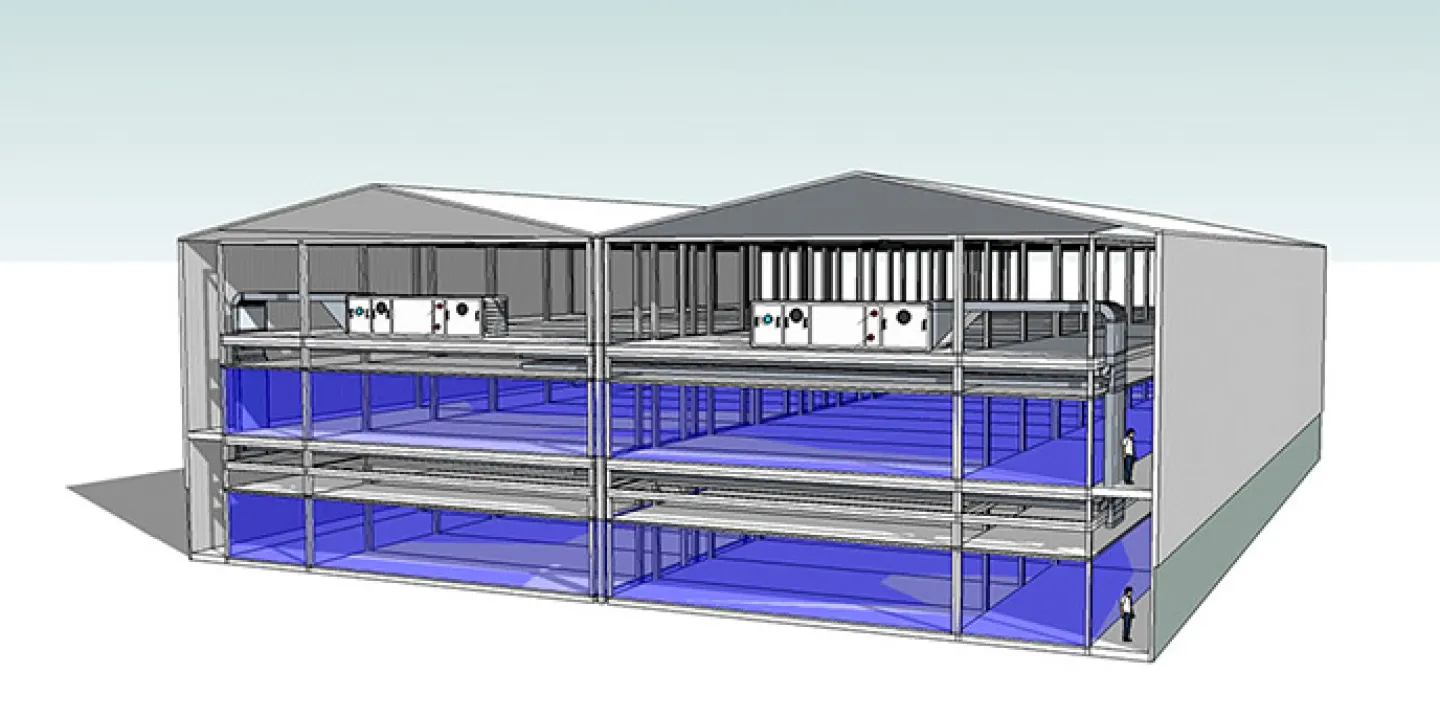
An indoor, modular oral solid dose (OSD) manufacturing facility on PiSA’s campus in Tlajomulco, Jalisco Province, Mexico is based on KeyPlants’ Conceptual Design and Basic Design solution. One requirement was for it to have high flexibility for multi-product manufacturing.
Our solution for tablets in blister was designed to meet Mexican, U.S., and EU GMP requirements and was designed to be executed in three main phases. KeyPlants won the contract through a referral by Telstar, a specialist in the development of engineering & construction projects, integrated process equipment, and GMP consultancy solutions.

Do you have any questions?
Do you have any questions?