Design References
Latin America, Biologics Drug Substance and Aseptic Formulation and Filling
Project Details
Project Details
Client
Confidential
Location
Latin America
Market
Biologics / Process Technology Installations
Solutions we provided
Turnkey delivery of two integrated process buildings in outdoor modules.
Project Overview
Project Overview
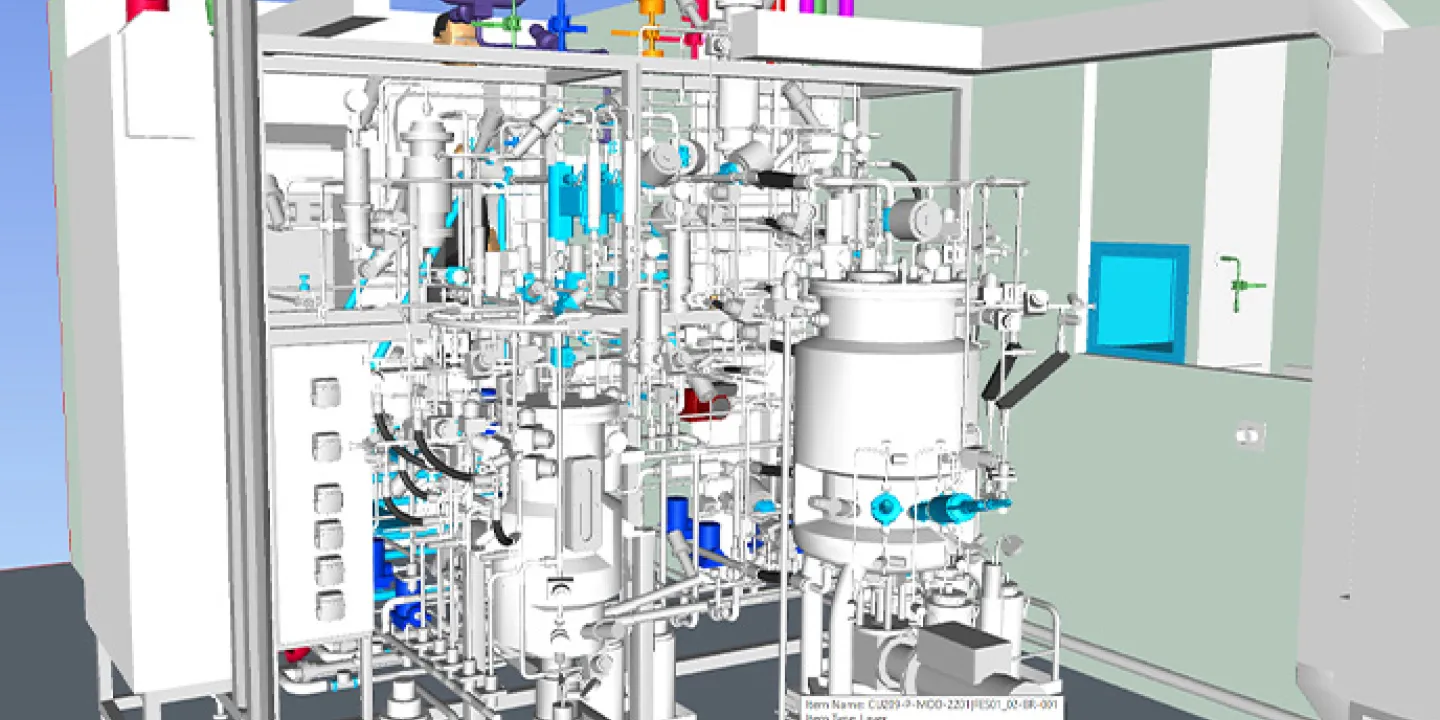
Our turnkey delivery of two integrated modular facilities for bulk manufacturing and aseptic filling of mAbs and therapetic proteins began operating in 2023 after several years of design and development work. The facilities are based on two of our design platforms for MBS (Modular Bio Solutions) and MAS (Modular Aseptic Solutions).
It is producing biosimilars and patented mAbs in three separate production buildings.
The project started with conceptual and basic design for the 6,300-sqm modular multi-product facility. We took the project all the way from concept to the final steps of commissioning on the client’s Latin American site.
Processes include bacterial fermentation with ultra-filtration and chromatography purification. The drug product area includes formulation, liquid filling with and without lyophilization, as well as powder filling in vials and filling of dual-chamber syringes.