31 May 2022
New pod facility from KeyPlants shortens lead times for pharma production to weeks
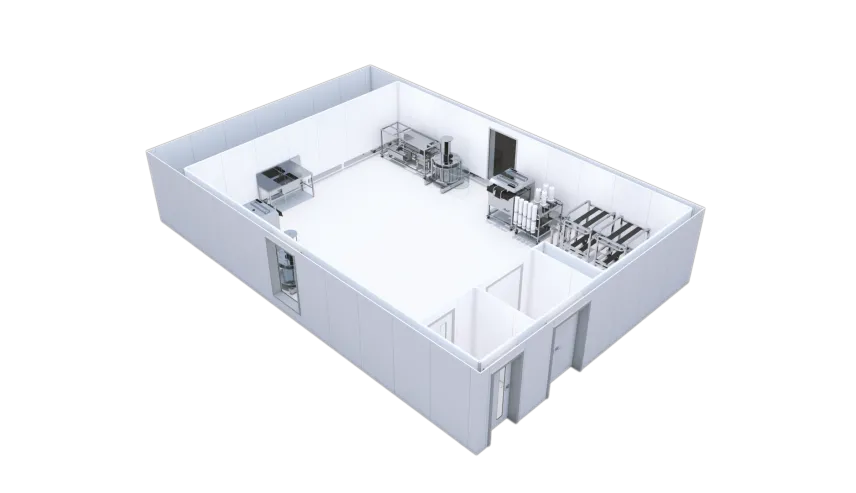
A new, portable on-demand (pod) facility will help pharma companies dramatically reduce the time it takes to set up new production, speed the development of cutting-edge therapies, and increase production flexibility. The product, called X-press pods, was developed by KeyPlants, the Stockholm, Sweden-based provider of modular prefabricated facility solutions.
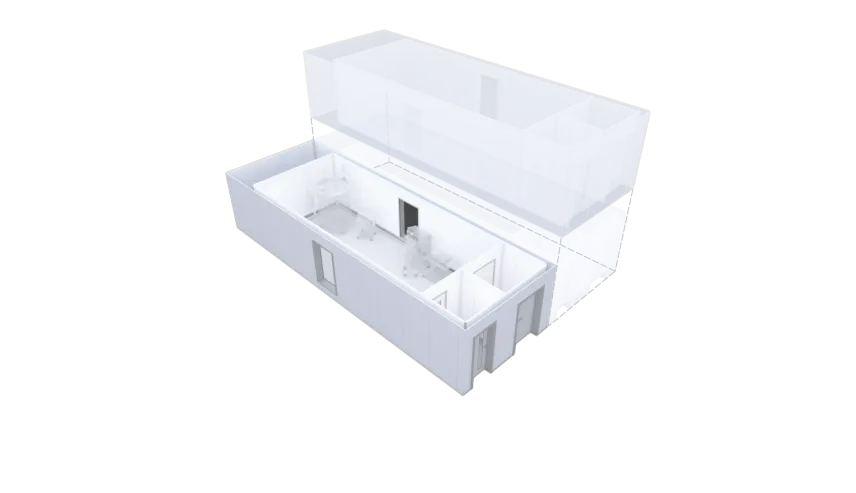
X-press pods can be on-site and ready for operations anywhere in the world in as few as five weeks for a single pod, compared to a year or more to build a conventional facility from scratch. It also offers maximum flexibility in production. “X-press pods give manufacturers the flexibility to start small-scale production immediately and then scale out as needed,” says Fredrik Eneqvist, Director Product Development, at KeyPlants. “You just add X-press pods instead of rebuilding. It’s the fastest way to start or expand production.”
X-press pods also offer the advantages of a fixed price and time schedule, with a turnkey, integrated approach instead of pieces of a facility/process engineering puzzle. “With X-press pods, we’re selling a product, not a project,” says KeyPlants’ Head of Sales, David Lindholm. “Pharmaceutical companies can eliminate the time and complexity of specification, bidding, procurement and permitting – to say nothing of supervising construction. This will result in innovative, cost-effective solutions from clinical development to commercialization since total cost of ownership will be reduced.”
With a standardized design, KeyPlants’ X-press solution allows for rapid lead times as short as five weeks when procuring a single X-press pod. X-press also ensures a facility that meets project timelines, without compromising on quality.
“The demand for capacity has increased, and the demand for flexible manufacturing has increased,” Lindholm says. “The demand for local manufacturing has also increased as pandemic-related supply-chain issues have created havoc in many industries around the world. Pharmaceuticals are no exception.”
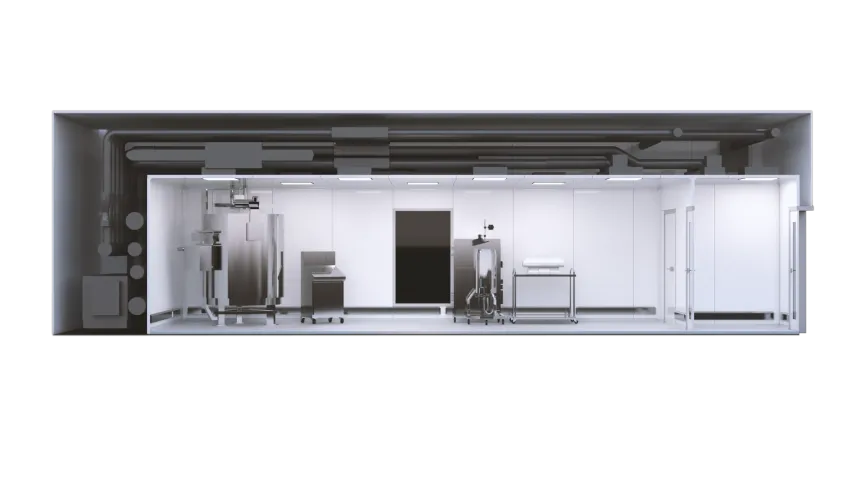
X-press pods offer manufacturers the flexibility to choose their own equipment or take advantage of KeyPlants’ capabilities. These capabilities include process design and equipment integration, as well as services to support with automation, design, qualification and process validation.
“X-press pods are technology agnostic,” Eneqvist adds. “Companies can change functionality by adding or removing walls or ramp up production by connecting additional pods.
Versatile, scalable, and always up to date
Eneqvist says that X-press pods can house many different processes across the whole pharmaceutical and biomanufacturing industries. “The speed with which new drugs and therapies are emerging in universities and research facilities all over the world means that a facility designed today might be obsolete a short time after it starts production,” he notes.
The pharmaceutical industry’s trend towards more personalized medicines such as ATMPs (Advanced Therapy Medicinal Products) also impacts the construction of plants.
“Traditional pharmaceutical and biomanufacturing companies typically have a large amount of experience and expertise in high-requirement production, such as aseptic conditions,” he explains. “ATMPs, on the other hand, are often at clinical stage and in the hands of researchers without the same experience in building and running production facilities.”
As these products move from the clinical stage to commercial, the owners face new challenges, but Lindholm says that KeyPlants, as a company that has built pharmaceutical manufacturing plants all over the world, has a thorough understanding of the technical and regulatory requirements, so the facilities will be set up for safe delivery of medicines to patients.
“With X-press pods, pharma companies can be confident that they will receive a facility that meets their project timelines, without compromising on quality.”
No limitation on scale
As a facility that can be up-sized by adding pods, X-press pods has no obvious limitations on its scale-up or scale-out capacity, opening up opportunities for future expansion. At the same time, X-press pods are also a solution for small-scale production of cell and gene therapies, for example.
“A large, conventional plant would not be cost-effective, given the small number of patients for some of these products,” Lindholm points out. “But X-press pods make it easy to right-size a facility.”
The portable nature of X-press pods also means they have the capacity to move from one location to another when required. “This ensures pods can adapt to evolving needs, which is crucial when keeping up with the fast-moving biomanufacturing landscape,” he adds.